Overview
We invite startups and university-backed projects to apply for non-dilutive funding ranging from $500,000 to $2,000,000 per project. This funding supports the advanced development of innovative digital health tools aligned with the Center for the Biomedical Advanced Research and Development Authority’s (BARDA) mission to advance products that assess traumatic brain injuries (TBI) caused by nuclear detonation and other blast events.
MATTER’s Paratus Digital Health Accelerator-BARDA Accelerator Network Hub for Digital Health provides funding and support to help entrepreneurs develop the clinical, technical and business aspects of their solutions. Paratus also connects innovators with mission-aligned study sites to accelerate development and validation.
Who should apply?
We are seeking innovators building digital health tools that assess TBI caused by nuclear detonation and other blast events in prehospital settings.
This global call welcomes companies, nonprofits and academic teams with proof of concept, evidence of demand or product–market fit and viable sustainability/commercialization potential. The base requirements for the program include the following:
-
General: Digital health technology related to diagnosis and triage of suspected traumatic brain injury, ideally for both adult and pediatric populations.
-
Technology maturity: At a minimum, data collection and analysis approach have been completed.
-
Software Prototype Stage: $500k - $1M; approximately 12 months long projects. Applicant has established their data collection and analysis approach, but additional software development, analytical validation and data collection from a small number of TBI patients is needed to build their algorithm and prepare for a larger-scale data collection.
-
Early Clinical Stage: Up to $2M; approximately 12-24 months long projects. Applicant has demonstrated proof-of-concept for their indication with a small number of TBI patients but expanded clinical data collection is needed to refine and test their algorithm, and to demonstrate the value of using their technology.
-
-
Intended use: The solution should be usable by healthcare professionals, first responders and/or self-administered within 24-72 hours following the injury in a prehospital setting, with limited access to imaging and lab equipment. Results in two to five minutes.
-
System/device architecture: Mobile medical application running on commonly available consumer technologies with limited auxiliary components. Compatible with popular operating systems and, ideally, interoperable with electronic medical record (EMR) systems.
For the full detail on program requirements, review BARDA’s official solicitation and outline of desired product attributes.
This program is conducted virtually.
Office hours
Have questions? Join us for office hours to learn more.
Submission process
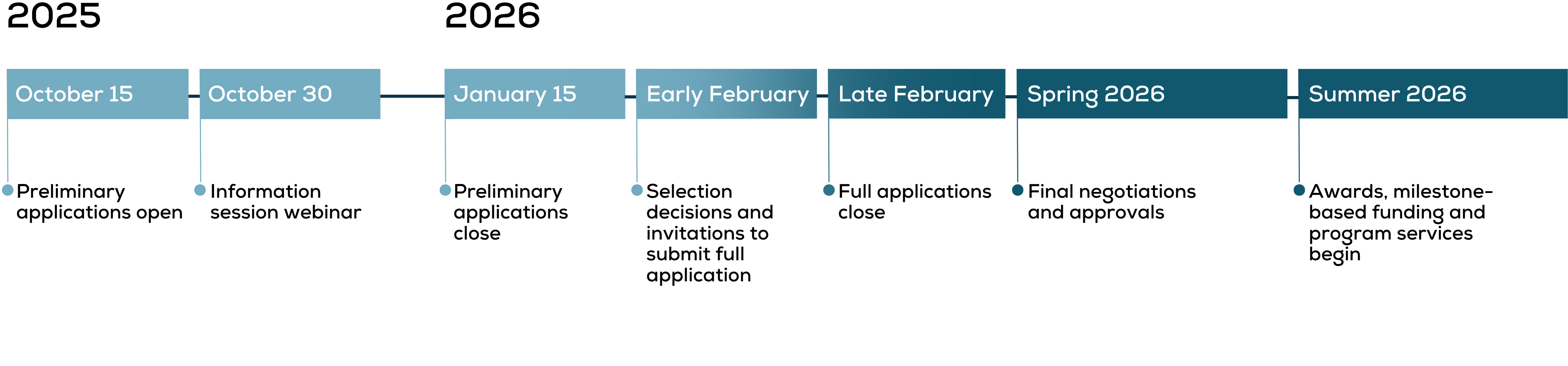
Preliminary application
Complete the preliminary application by January 15, 2026.
Invitation to submit
Selected applicants will be invited to submit full proposals, including milestones and budgets, by February 2026.
Notification of acceptance
The first round of awards will be announced in June 2026.
Non-dilutive funding awards
Milestone-based funding and program services begin in summer 2026.
Project formats
Applicants can submit applications for Software Prototype Stage or Early Clinical Stage funding with a timeline of up to 24 months, within a range from $500,000 to $2,000,000.
Funding uses
- Engage a study site to complete validation work
- Conduct a clinical study to demonstrate technical capability of solution
- Advance technologies currently in development to the next milestone or value inflection point
- Engage with FDA through the Q-submission pathway
- Funding is not intended for device and hardware development
Program deliverables to the sponsor include a final report, a software prototype and a complete de-identified dataset.
Study pathways
The program uses structured study pathways led by a principal investigator and defined staff for full mentoring and oversight. Each study has a clear business or clinical question and is not a pilot or preliminary IRB project. Companies are matched with industry partners based on specific technology and clinical needs.
Studies last up to 24 months and focus on clinical demonstration, real-world evidence and/or technology assessment.
We maintain a curated network of health system and industry partners, each pre-qualified through rigorous assessment of their clinical capabilities, research infrastructure and regulatory compliance. Our matching process systematically aligns company technologies with appropriate partner organizations based on expertise, patient population access and institutional research capacity. Partner selection criteria include demonstrated experience with medical device studies, established IRB processes and dedicated clinical research staff. The program facilitates formal partnership agreements that define roles, responsibilities and intellectual property arrangements, ensuring seamless integration of company objectives with partner capabilities while maintaining compliance with all regulatory and ethical standards.
Benefits of participation
Startups and studies will receive project management support over the award duration. Additional benefits of participation include the following:
- Non-dilutive funding to complete a time-bound development, evaluation or validation study around their concept
- Mentorship from industry leaders and subject matter experts
- Exposure to potential investors, partners and clients
Timeline
Gain of function research
This funding opportunity does not support gain of function research or research that involves the manipulation of pathogens resulting in potential gain of function.
Proposals will be evaluated for the appropriate use of strain(s) in proposed studies.
See the Non-dilutive Funding for Traumatic Brain Injury Assessment FAQs for more information.
Explore more opportunities with Paratus
About MATTER
At MATTER, we believe collaboration is the best way to improve healthcare. The MATTER collaborative includes more than 1,000 current and alumni startups from around the world, working together with dozens of hospitals and health systems, universities and industry-leading companies to build the future of healthcare. Together we are accelerating innovation, advancing care and improving lives. For more information, visit matter.health and follow @MATTERhealth.
The BARDA Accelerator Network
The BARDA Accelerator Network aims to provide comprehensive support to health security innovators, startups and BARDA portfolio companies through wrap-around accelerator services, technical and business/commercialization expertise, and resources. The network will facilitate the rapid development, evaluation, validation and commercialization of medical countermeasures.






